

NCT04155749: Phase 1 : CART-ddBCMA - Master Protocol for Cell Therapies in Multiple Myeloma
NCT04155749: Phase 1: Master Protocol for the Phase 1 Study of Cell Therapies in Multiple Myeloma Master Protocol for the Phase 1 Study...
Dec 5, 2019
389


NCT03941860: Phase 3 - EAA171 - OPTIMUM - Addition of Ixazomib to Lenalidomide in Residual Myeloma
EAA171 OPTIMUM NCT03941860: Testing the Addition of Ixazomib to Lenalidomide in Patients With Evidence of Residual Multiple Myeloma,...
Dec 5, 2019
278


NCT03651128: Phase 3 - Study of bb2121 Vs Standard Regimens Refractory Myeloma (RRMM) (KarMMa-3)
KarMMa-3 bb2121 Efficacy and Safety Study of bb2121 Versus Standard Regimens in Subjects With Relapsed and Refractory Multiple Myeloma...
Dec 16, 2018
578


NCT03710603: Phase 3 - Daratumumab, VELCADE, Len, Dex VS VELCADE, Len, Dex in NDMM (Perseus) EMN17
Perseus Study NDMM Newly Diagnosed Multiple Myeloma EMN 17 Daratumumab, VELCADE (Bortezomib), Lenalidomide and Dexamethasone Compared to...
Dec 15, 2018
1,690


NCT03729804: Phase 3: KRd Versus VRd in Patients With Newly Diagnosed Multiple Myeloma (COBRA)
NCT03729804: Phase 3: Carfilzomib, Lenalidomide, and Dexamethasone Versus Bortezomib, Lenalidomide and Dexamethasone (KRd vs. VRd) in...
Dec 13, 2018
137


NCT03606577: Phase 2-IFM 2018 - 04 - Quadruplet Induction & Consolidation + Tandem ASCT Hi Risk NDMM
IFM 2018-04 NCT03606577: Phase 2: An Intensive Program With Quadruplet Induction and Consolidation Plus Tandem Autologous Stem Cell...
Dec 13, 2018
512


NCT03617731: Phase 3 - Effect of Isatuximab to RVd Induction and Len Maintenance in NDMM (GMMG HD7)
NCT03617731: Phase 3 - Effect of Isatuximab to RVd Induction and Lenalidomide Maintenance in NDMM (GMMG HD7) German-speaking myeloma...
Dec 6, 2018
259


RADAR (UK-MRA Myeloma XV): treatment escalation and de-escalation strategies in NDMM - TE Myeloma
RADAR (UK-MRA Myeloma XV): Risk-Adapted therapy Directed According to Response comparing treatment escalation and de-escalation...
Dec 6, 2018
271


NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose Dexamethasone (DKRd) NDMM
Multiple Myeloma Research Consortium phase 2 study Dara-KRd NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose...
Dec 5, 2018
211


NCT03720041: Phase 3 Myeloma XIV: Frailty-adjusted Therapy in Transplant Non-Elig. New MM (FiTNEss)
Myeloma XIV: Frailty-adjusted Therapy in Transplant Non-Eligible Patients With Newly Diagnosed Multiple Myeloma (FiTNEss) FiTNEss (UK-MRA...
Dec 3, 2018
353

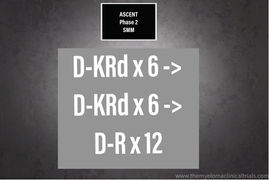
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
435


NCT03224507: Phase 2 - Monoclonal Antibody-Based Sequential Therapy for Deep Remission in MM -MASTER
MASTER TRIAL Monoclonal Antibody-Based Sequential Therapy for Deep Remission in Multiple Myeloma (MASTER) Dara-KRd Monoclonal...
Dec 1, 2017
434


NCT02874742 : Phase 2 - Daratumumab, Len., Bortezomib & Dex (D-RVd) Vs (RVd) in New MM - GRIFFIN
The GRIFFIN trial Study Comparing Daratumumab, Lenalidomide, Bortezomib, and Dexamethasone (D-RVd) Versus Lenalidomide, Bortezomib, and...
Dec 31, 2016
713


NCT02659293: Phase 3 - ATLAS Trial: Carfilzomib, Lenalidomide & Dex Vs Lenalidomide Alone After ASCT
NCT02659293: Phase 3: ATLAS Trial: Carfilzomib, Lenalidomide and Dex Vs Lenalidomide Alone After ASCT KRd Vs R NDMM Trial of Carfilzomib,...
Dec 30, 2016
352


NCT02969837: Phase 2 - Study of Initial Treatment With Elotuzumab, Carfilzomib, Len and Dex NDMM
NCT02969837: Phase 2 - Study of Initial Treatment With Elotuzumab, Carfilzomib, Lenalidomide and Dexamethasone in Multiple Myeloma...
Dec 7, 2016
174


NCT02076009: Phase 3 - Daratumumab, Lenalidomide & Dex (DRd) VS Len & Dexa (Rd) - MMY3003 (POLLUX)
MMY3003 (POLLUX) Study NCT02076009: Phase 3: A Study Comparing Daratumumab, Lenalidomide, and Dexamethasone With Lenalidomide and...
Dec 19, 2014
394


NCT02195479 : A Phase 3, Study of Bortezomib, Melphalan, Prednisone & Daratumumab ALCYONE trial
ALCYONE trial NCT02195479: Phase 3: A Study of Combination of Daratumumab and Velcade (Bortezomib) Melphalan-Prednisone (DVMP) Compared...
Dec 12, 2014
310


NCT02252172 : Phase 3 - Dara, Lena & Dex VS Len & Dex in previously untreated Myeloma - MAIA STUDY
The MAIA Study NCT02252172: Phase 3: Study Comparing Daratumumab, Lenalidomide, and Dexamethasone With Lenalidomide and Dexamethasone in...
Dec 12, 2014
497


NCT02136134: Phase 3 - Daratumumab Plus Bortezomib & Dexa in relapsed Myeloma MMY3004 (CASTOR) Study
The CASTOR Trial Dara Vd DVd NCT02136134: Phase 3: Addition of Daratumumab to Combination of Bortezomib and Dexamethasone in Participants...
Dec 11, 2014
509

