

NCT03412565: Phase 2 - Subcutaneous Daratumumab in Combination With Standard MM Regimens - PLEIADES
PLEIADES study A Study to Evaluate Subcutaneous Daratumumab in Combination With Standard Multiple Myeloma Treatment Regimen MMY2040...
Dec 14, 2018
634


NCT03729804: Phase 3: KRd Versus VRd in Patients With Newly Diagnosed Multiple Myeloma (COBRA)
NCT03729804: Phase 3: Carfilzomib, Lenalidomide, and Dexamethasone Versus Bortezomib, Lenalidomide and Dexamethasone (KRd vs. VRd) in...
Dec 13, 2018
137


NCT03606577: Phase 2-IFM 2018 - 04 - Quadruplet Induction & Consolidation + Tandem ASCT Hi Risk NDMM
IFM 2018-04 NCT03606577: Phase 2: An Intensive Program With Quadruplet Induction and Consolidation Plus Tandem Autologous Stem Cell...
Dec 13, 2018
512


NCT03673826: Phase 2: Carfilzomib, Lenalidomide and Dex Versus Lenalidomide and Dex High- Risk SMM
HO147SMM NCT03673826: Phase 2: Carfilzomib, Lenalidomide and Dexamethasone Versus Lenalidomide and Dexamethasone in High- Risk SMM...
Dec 8, 2018
101


NCT03556332: Phase 2 - Carfilzomib, Lenalidomide, Dex Daratumumab for relap. MM with auto transplant
A Study of Carfilzomib, Lenalidomide, Dexamethasone and Daratumumab for Patients With Relapsed/Refractory Myeloma With Salvage Autologous...
Dec 7, 2018
239


NCT01554852: Phase 3- Thalidomide, Lenalidomide, Carfilzomib, Bortezomib, Vorinostat-NDMM Myeloma XI
Myeloma XI The purpose of this study is to compare a standard chemotherapy regimen of cyclophosphamide, dexamethasone plus thalidomide...
Dec 7, 2018
399


NCT03617731: Phase 3 - Effect of Isatuximab to RVd Induction and Len Maintenance in NDMM (GMMG HD7)
NCT03617731: Phase 3 - Effect of Isatuximab to RVd Induction and Lenalidomide Maintenance in NDMM (GMMG HD7) German-speaking myeloma...
Dec 6, 2018
259


RADAR (UK-MRA Myeloma XV): treatment escalation and de-escalation strategies in NDMM - TE Myeloma
RADAR (UK-MRA Myeloma XV): Risk-Adapted therapy Directed According to Response comparing treatment escalation and de-escalation...
Dec 6, 2018
272


NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose Dexamethasone (DKRd) NDMM
Multiple Myeloma Research Consortium phase 2 study Dara-KRd NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose...
Dec 5, 2018
212


NCT03702725: Phase 1: Study of Ibrutinib in Combination With Revlimid/Dexamethasone in RRMM Myeloma
NCT03702725: Phase 1: Study of Ibrutinib in Combination With Revlimid/Dexamethasone in Relapsed/Refractory Multiple Myeloma Study of...
Dec 4, 2018
49


NCT03720041: Phase 3 Myeloma XIV: Frailty-adjusted Therapy in Transplant Non-Elig. New MM (FiTNEss)
Myeloma XIV: Frailty-adjusted Therapy in Transplant Non-Eligible Patients With Newly Diagnosed Multiple Myeloma (FiTNEss) FiTNEss (UK-MRA...
Dec 3, 2018
353


NCT03290950: Phase 2 - A Study of Daratumumab in Patients With NDMM wKRd-D - Manhattan Trial
MANHATTAN TRIAL NCT03290950: Phase 2 - A Study of Daratumumab in Patients With Newly Diagnosed Multiple Myeloma Dara-KRd A Study of...
Dec 31, 2017
790


NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy RRMM Myeloma
NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy for Relapsed Multiple Myeloma Testing the Addition...
Dec 26, 2017
64


Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone
Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone in multiple myeloma: A systematic review...
Dec 22, 2017
24

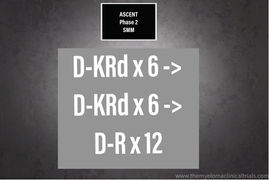
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
435


NCT03319667: Phase 3 - Isatuximab, Bortezomib, Lenalidomide and Dexamethasone NDMM (IMROZ)
IMROZ Study NCT03319667 Clinical Benefit of SAR650984, Bortezomib, Lenalidomide and Dexamethasone Combination in NDMM Patients Not...
Dec 16, 2017
662


NCT03106324: Safety Study of Lenalidomide in Previously Untreated Adult Multiple Myeloma -TNE
NCT03106324: Safety Study of Lenalidomide in Previously Untreated Adult Multiple Myeloma Patients Who Are Not Eligible for Transplant A...
Dec 15, 2017
67


NCT03104842: Phase 2 - GMMG - CONCEPT - Eval. iNduction, Consol. & Maint. Isatuximab Carf Len. & Dex
GMMG - The German Speaking Myeloma Multicenter Group GMMG-CONCEPT trial Isa-KRd NCT03104842: Phase 2 - Evaluation iNduction,...
Dec 14, 2017
267


NCT03269136: Phase 1 -PF-06863135 As Single Agent & with Immunomodulatory Agents In relapsed Myeloma
NCT03269136 MAGNETISMM-1 RRMM NCT03269136: Phase 1 -PF-06863135 As Single Agent & with Immunomodulatory Agents In relapsed Myeloma To...
Dec 9, 2017
506

