

NCT04935580: Phase 1/2: Study of FasT CAR-T GC012F Injection in High Risk TE NDMM Patients
NCT04935580: Phase 1/2: Study of FasT CAR-T GC012F Injection in High Risk TE NDMM Patients Study of FasT CAR-T GC012F Injection in High...
Jun 23, 2021
348


NCT04674813: Phase 1 - A Study of CC-95266 in Subjects With Relapsed and/or Refractory Myeloma
A Study of CC-95266 in Subjects With Relapsed and/or Refractory Multiple Myeloma This is a Phase 1, multicenter, open label, study of...
Dec 27, 2020
281


NCT04555551:Phase 1 - MCARH109 Chimeric Antigen Receptor (CAR) Modified T Cells for Multiple Myeloma
MCARH109 CART MCARH109 Chimeric Antigen Receptor (CAR) Modified T Cells for the Treatment of Multiple Myeloma This study will test the...
Dec 17, 2020
700


NCT04093596: Phase 1 - ALLO-715 BCMA Allogenic CAR T Cells in relapsed Multiple Myeloma (UNIVERSAL)
UNIVERSAL Safety and Efficacy of ALLO-715 BCMA Allogenic CAR T Cells in in Adults With Relapsed or Refractory Multiple Myeloma...
Dec 12, 2020
420


NCT04309981: Phase 1/2: Humanized CART Directed Against BCMA (ARI0002h) in RRMM Multiple Myeloma
NCT04309981: Phase 1/2: Humanized CART Directed Against BCMA (ARI0002h) in RRMM to Proteasome Inhibitors, Immunomodulators and Anti-CD38...
Dec 12, 2020
222


NCT04244656: Phase 1 -A Safety and Efficacy Study Evaluating CTX120 Relapsed Refractory Myeloma
NCT04244656: Phase 1 -A Safety and Efficacy Study Evaluating CTX120 in Subjects With Relapsed or Refractory Multiple Myeloma This is a...
Dec 11, 2020
176

NCT04394650: Phase 1 - CC-98633, BCMA-targeted Chimeric Antigen Receptor (CAR) T Cells, in RRMM
CC-98633 CART NCT04394650: Phase 1 - A Study of CC-98633, BCMA-targeted Chimeric Antigen Receptor (CAR) T Cells, in Subjects With...
Dec 1, 2020
207


NCT04236011: Phase 1: BCMA and CD19 Targeted Fast Dual CAR-T for BCMA+ Refractory/Relapsed Myeloma
NCT04236011: Phase 1: BCMA and CD19 Targeted Fast Dual CAR-T for BCMA+ Refractory/Relapsed Multiple Myeloma BCMA and CD19 Targeted Fast...
Dec 1, 2020
329


NCT04499339: Phase 1/2: Autologous SLAMF7 CAR-T Cells in Multiple Myeloma - RRMM - CARAMBA-1
CARAMBA-1 - NCT04499339: Phase 1/2: Autologous SLAMF7 CAR-T Cells in Multiple Myeloma CARAMBA-1 - NCT04499339: Phase 1/2: Autologous...
Dec 1, 2020
36


NCT04318327: Phase 1 - BCMA-directed CAR-T Cell Therapy in Adult Patients With RRMM
NCT04318327: Phase 1 - BCMA-directed CAR-T Cell Therapy in Adult Patients With Relapsed and/or Refractory Multiple Myeloma This is a...
Dec 1, 2020
206


NCT04181827: Phase 3 -Cilta-cel vs Pom/Bort/Dex or Dara/Pom/Dex in relpsd. len ref MM CARTITUDE 4
CARTITUDE-4 Ciltacabtagene Autoleucel A Study Comparing JNJ-68284528, a CAR-T Therapy Directed Against B-cell Maturation Antigen (BCMA),...
Dec 28, 2019
1,432

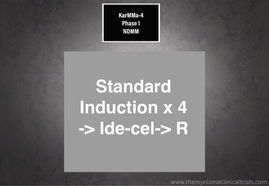
NCT04196491: Phase 1 - Evaluate the Safety of bb2121 in High Risk, New Myeloma (NDMM) (KarMMa-4)
KarMMa-4 A Study to Evaluate the Safety of bb2121 in Subjects With High Risk, Newly Diagnosed Multiple Myeloma (NDMM) (KarMMa-4) Relapsed...
Dec 27, 2019
575


NCT04133636: Phase 2 - Study of JNJ-68284528, CAR-T Therapy Directed Against BCMA in MM -CARTITUDE-2
CARTITUDE-2 Ciltacabtagene Autoleuce A Study of JNJ-68284528, a Chimeric Antigen Receptor T Cell (CAR-T) Therapy Directed Against B-cell...
Dec 19, 2019
779


NCT03915184: Phase 1 - Clinical Trial to Evaluate CT053 in Patients With Relapsed and/or Refractory
(LUMMICAR STUDY 2) NCT03915184: Phase 1 - Clinical Trial to Evaluate CT053 in Patients With Relapsed and/or Refractory Multiple Myeloma...
Dec 13, 2019
372


NCT03975907: Phase 1/2 - Clinical Trial to Evaluate CT053 in Patients With RRMM (LUMMICAR STUDY 1)
(LUMMICAR STUDY 1) NCT03975907: Phase 1/2 - Clinical Trial to Evaluate CT053 in Patients With Relapsed and/or Refractory Multiple Myeloma...
Dec 6, 2019
104


NCT04155749: Phase 1 : CART-ddBCMA - Master Protocol for Cell Therapies in Multiple Myeloma
NCT04155749: Phase 1: Master Protocol for the Phase 1 Study of Cell Therapies in Multiple Myeloma Master Protocol for the Phase 1 Study...
Dec 5, 2019
390


NCT04182581: Phase 1: A Study of BCMA/CD19 Dual-Target CAR-T Cell Immunotherapy for Relapsed Myeloma
NCT04182581: Phase 1: A Study of BCMA/CD19 Dual-Target CAR-T Cell Immunotherapy for Relapsed or Refractory Multiple Myeloma A Study of...
Dec 2, 2019
96


NCT03549442: Phase 1 - CART-BCMA +/- huCART19 as Consolidation High Risk MM
RRMM Phase A: Safety Run-in to test the safety of CART-BCMA + huCART19 as split-dose infusions after lymphodepleting chemotherapy with...
Dec 27, 2018
508


NCT03548207: Phase 1b -2 - JNJ-68284528, CAR-T Therapy against BCMA in relapsed MM (CARTITUDE-1)
CARTITUDE-1 Ciltacabtagene Autoleucel autologous bi-epitope BCMA-targeted CAR T cells JNJ-68284528 LCAR-B38M cilta-cel The purpose of the...
Dec 26, 2018
690

