

NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose Dexamethasone (DKRd) NDMM
Multiple Myeloma Research Consortium phase 2 study Dara-KRd NCT03500445: Phase 2: Daratumumab, Carfilzomib, Lenalidomide and Low Dose...
Dec 5, 2018
216


NCT03530683: Phase 1: A Trial of TTI-622 in Patients With Advanced Hem. Malignancies (TTI-622-01)
NCT03530683: Phase 1: A Trial of TTI-622 in Patients With Advanced Hematologic Malignancies (TTI-622-01) Sponsor Trillium Therapeutics...
Dec 4, 2018
543


NCT03290950: Phase 2 - A Study of Daratumumab in Patients With NDMM wKRd-D - Manhattan Trial
MANHATTAN TRIAL NCT03290950: Phase 2 - A Study of Daratumumab in Patients With Newly Diagnosed Multiple Myeloma Dara-KRd A Study of...
Dec 31, 2017
795


NCT03158688: Phase 3 - Carfilzomib, Daratumumab and Dexa for relapsed Multiple Myeloma. (CANDOR)
Phase 3 CANDOR study Dara-Kd Kd Study of Carfilzomib, Daratumumab and Dexamethasone for Patients With Relapsed and/or Refractory Multiple...
Dec 28, 2017
343


NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy RRMM Myeloma
NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy for Relapsed Multiple Myeloma Testing the Addition...
Dec 26, 2017
67

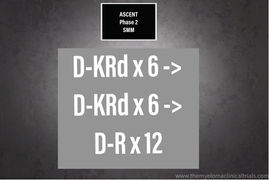
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
442


NCT03104842: Phase 2 - GMMG - CONCEPT - Eval. iNduction, Consol. & Maint. Isatuximab Carf Len. & Dex
GMMG - The German Speaking Myeloma Multicenter Group GMMG-CONCEPT trial Isa-KRd NCT03104842: Phase 2 - Evaluation iNduction,...
Dec 14, 2017
267


NCT03275285: Phase 3 - Isatuximab, Carf & Dex VS Carf And Dex In Relapse Multiple Myeloma (IKEMA)
IKEMA Isa-Kd Vs Kd Multinational Clinical Study Comparing Isatuximab, Carfilzomib And Dexamethasone To Carfilzomib And Dexamethasone In...
Dec 10, 2017
474


NCT03336073: Phase 2: GEM-KyCyDex - Carfilzomib, Cyclophosphamide, Dexamethasone in Multiple Myeloma
NCT03336073: Phase 2: GEM-KyCyDex - Carfilzomib, Cyclophosphamide, Dexamethasone in Multiple Myeloma This is a multicenter, open label,...
Dec 1, 2017
68


NCT03224507: Phase 2 - Monoclonal Antibody-Based Sequential Therapy for Deep Remission in MM -MASTER
MASTER TRIAL Monoclonal Antibody-Based Sequential Therapy for Deep Remission in Multiple Myeloma (MASTER) Dara-KRd Monoclonal...
Dec 1, 2017
435


NCT02659293: Phase 3 - ATLAS Trial: Carfilzomib, Lenalidomide & Dex Vs Lenalidomide Alone After ASCT
NCT02659293: Phase 3: ATLAS Trial: Carfilzomib, Lenalidomide and Dex Vs Lenalidomide Alone After ASCT KRd Vs R NDMM Trial of Carfilzomib,...
Dec 30, 2016
352


NCT02899052: Phase 2: Study of Venetoclax in Combination With Carfilzomib and Dexamethasone in RRMM
NCT02899052: Phase 2: Study of Venetoclax in Combination With Carfilzomib and Dexamethasone in Participants With Relapsed or Refractory...
Dec 16, 2016
97


NCT02891811: Phase 2: Patients With Newly Diagnosed Multiple Myeloma Comparing KTd vs. KRd Induction
NCT02891811: Phase 2: Patients With Newly Diagnosed Multiple Myeloma Comparing KTd vs. KRd Induction Therapy and Investigating a K-mono...
Dec 13, 2016
92


NCT02969837: Phase 2 - Study of Initial Treatment With Elotuzumab, Carfilzomib, Len and Dex NDMM
NCT02969837: Phase 2 - Study of Initial Treatment With Elotuzumab, Carfilzomib, Lenalidomide and Dexamethasone in Multiple Myeloma...
Dec 7, 2016
174


NCT02332850: Phase Ib Study of SAR650984 With Carfilzomib for Treatment of Relapsed Multiple Myeloma
Multiple Myeloma Research Consortium NCT02332850: Phase Ib Study of SAR650984 With Carfilzomib for Treatment of Relapsed Multiple Myeloma...
Dec 23, 2015
175


NCT02415413: Phase 2 - Carfilzomib in Patients Under 65 With High Risk Smoldering MM GEM-CESAR trial
GEM-CESAR trial PETHEMA/GEM Spanish Myeloma Group GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en...
Dec 19, 2015
471


Ph 2: Carfilzomib & lenalidomide-based treatment for newly diagnosed primary plasma cell leukemia
EMN12/HOVON129 Study Phase 2: To evaluate progression-free survival in adult pPCL patients by incorporation of carfilzomib and...
Dec 9, 2015
111


NCT02412878: Phase 3 - Once-weekly vs Twice-weekly Carfilzomib With Dexa in Relapsed MM (ARROW)
Phase 3 A.R.R.O.W. trial - ARROW Kd The purpose of the study is to compare the progression-free survival (PFS) of once-weekly carfilzomib...
Dec 4, 2015
274


NCT02199665: Phase 1: Selinexor, Carfilzomib, & Dexamethasone in Treating Patients With RRMM (SINE)
Multiple Myeloma Research Consortium NCT02199665: Phase 1: Selinexor, Carfilzomib, and Dexamethasone in Treating Patients With Relapsed...
Dec 13, 2014
112

