

NCT03631043: Phase 1 - Personalized Vaccine in Treating Participants With Smoldering MM
Personalized Vaccine in Treating Patients With Smoldering Multiple Myeloma This early phase I trial studies the side effects of...
Dec 31, 2018
335


NCT03657251: MMRF CureCloud Research Initiative
NCT03657251: MMRF CureCloud Research Initiative NDMM RRMM MGUS/SMM MMRF CureCloud Research Initiative Observational Study The MMRF...
Dec 15, 2018
218


NCT03673826: Phase 2: Carfilzomib, Lenalidomide and Dex Versus Lenalidomide and Dex High- Risk SMM
HO147SMM NCT03673826: Phase 2: Carfilzomib, Lenalidomide and Dexamethasone Versus Lenalidomide and Dexamethasone in High- Risk SMM...
Dec 8, 2018
101

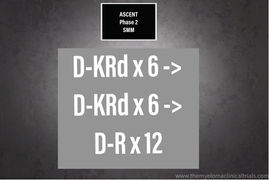
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
442


NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab MGUS / Smoldering Myeloma SMM
NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab in Patients With High-Risk MGUS and Low-Risk Smoldering Multiple...
Dec 15, 2017
180


NCT03301220: Phase 3: A Study of Subcutaneous Daratumumab Versus Active Monitoring in HI Risk SMM
NCT03301220: Phase 3: A Study of Subcutaneous Daratumumab Versus Active Monitoring in Participants With High-Risk Smoldering Multiple...
Dec 7, 2017
158


NCT03327597 : Iceland Screens, Treats or Prevents Multiple Myeloma (iStopMM)
Iceland Screens, Treats or Prevents Multiple Myeloma (iStopMM) Iceland Screens, Treats or Prevents Multiple Myeloma (iStopMM) This study...
Dec 2, 2017
390


NCT02960555: Phase 2 - Isatuximab in Patients With High Risk Smoldering Plasma Cell Myeloma
Isatuximab in Treating Patients With High Risk Smoldering Plasma Cell Myeloma This phase II trial studies how well isatuximab works in...
Dec 30, 2016
275


NCT02916771: Phase 2: Trial of Combination of Ixazomib and Lenalidomide and Dexamethasone in SMM
NCT02916771: Phase 2: Trial of Combination of Ixazomib and Lenalidomide and Dexamethasone in Smoldering Multiple Myeloma Trial of...
Dec 9, 2016
86


NCT02886065: Phase 1 - PVX-410, a Cancer Vaccine, & Citarinostat +/- Lenalidomide for Smoldering MM
NCT02886065: Phase 1 - PVX-410, a Cancer Vaccine, and Citarinostat +/- Lenalidomide for Smoldering MM A Study of PVX-410, a Cancer...
Dec 9, 2016
202


NCT02415413: Phase 2 - Carfilzomib in Patients Under 65 With High Risk Smoldering MM GEM-CESAR trial
GEM-CESAR trial PETHEMA/GEM Spanish Myeloma Group GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en...
Dec 19, 2015
471


NCT02316106: Phase 2 - A Study to Evaluate 3 Dose Schedules of Daratumumab in SMM - CENTAURUS
CENTAURUS A Study to Evaluate 3 Dose Schedules of Daratumumab in Participants With Smoldering Multiple Myeloma The purpose of this study...
Dec 12, 2014
171


NCT02279394: E-PRISM: Phase II Trial of Elotuzumab Lenalidomide and Dexamethasone in Hi R SMM
NCT02279394: E-PRISM: Phase II Trial of Elotuzumab Lenalidomide and Dexamethasone in High-Risk Smoldering Multiple Myeloma E-PRISM: Phase...
Dec 11, 2014
145


NCT01572480: Phase 2: Carfilzomib, Lenalidomide, and Dexamethasone for Smoldering Multiple Myeloma
NCT01572480: Phase 2: Carfilzomib, Lenalidomide, and Dexamethasone for Smoldering Multiple Myeloma KRd Multiple myeloma is a blood cancer...
Dec 23, 2012
183


NCT01169337: Phase 3 -Lenalidomide or Observation in Asymptomatic High-Risk Smoldering Myeloma E3A06
ECOG E3A06 ECOG-ACRIN Cancer Research Group ECOG E3A06 Lenalidomide or Observation in Treating Patients With Asymptomatic High-Risk...
Dec 10, 2010
226


NCT00480363: Phase 3: Revlimid / Dex ( ReDex) VS Observation in Smoldering MM (QUIREDEX)
QUIREDEX PETHEMA/GEM Spanish Myeloma Group GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en...
Dec 7, 2007
294

