

NCT03589222: Phase 2: SELIBORDARA: Selinexor, Bortezomib and Daratumumab in Multiple Myeloma
NCT03589222: Phase 2: SELIBORDARA: Selinexor, Bortezomib and Daratumumab in Multiple Myeloma NCT03589222: Phase 2: SELIBORDARA:...
Dec 1, 2018
35


NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy RRMM Myeloma
NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy for Relapsed Multiple Myeloma Testing the Addition...
Dec 26, 2017
67

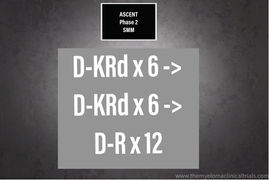
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
442


NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed Refractory Myeloma
Multiple Myeloma Research Consortium NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed and/or Refractory...
Dec 15, 2017
131


NCT03275103: Phase 1 - Dose-Escalation Study of Cevostamab in Participants With RRMM
NCT03275103: Phase 1 - Dose-Escalation Study of Cevostamab in Participants With Relapsed or Refractory Multiple Myeloma (R/R MM) A Phase...
Dec 14, 2017
357


NCT03145181: Phase 1 - Teclistamab, Humanized BCMA*CD3 Bispecific Ab, in Relapsed Myeloma MajesTEC1
MajesTEC1 Dose Escalation Study of Teclistamab, a Humanized BCMA*CD3 Bispecific Antibody, in Participants With Relapsed or Refractory...
Dec 12, 2017
385


NCT03078452: Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy - Multiple Myeloma
NCT03078452: Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy Evaluating Effectiveness of Powered Drill Bone Marrow Biopsy...
Dec 9, 2017
64


NCT03309111: Phase 1: ISB 1342, a CD38/CD3 Bispecific Antibody, in Subjects With RRMM
NCT03309111: Phase 1: ISB 1342, a CD38/CD3 Bispecific Antibody, in Subjects With Previously Treated Multiple Myeloma Study of ISB 1342, a...
Dec 6, 2017
209


NCT02671448: Pilot Trial of Homebound Stem Cell Transplantation
NCT02671448: Pilot Trial of Homebound Stem Cell Transplantation NCT02671448: Pilot Trial of Homebound Stem Cell Transplantation In this...
Dec 17, 2016
63


NCT02899052: Phase 2: Study of Venetoclax in Combination With Carfilzomib and Dexamethasone in RRMM
NCT02899052: Phase 2: Study of Venetoclax in Combination With Carfilzomib and Dexamethasone in Participants With Relapsed or Refractory...
Dec 16, 2016
97


NCT02833610: Phase 2 - A Study of Denosumab in Multiple Myeloma Patients With Renal Insufficiency
NCT02833610: Phase 2 - A Study of Denosumab in Multiple Myeloma Patients With Renal Insufficiency This research study is studying a...
Dec 9, 2016
149


NCT02693535: Phase 2: TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs
NCT02693535: Phase 2: TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a...
Dec 8, 2016
95


NCT02332850: Phase Ib Study of SAR650984 With Carfilzomib for Treatment of Relapsed Multiple Myeloma
Multiple Myeloma Research Consortium NCT02332850: Phase Ib Study of SAR650984 With Carfilzomib for Treatment of Relapsed Multiple Myeloma...
Dec 23, 2015
175


Ph 2: Carfilzomib & lenalidomide-based treatment for newly diagnosed primary plasma cell leukemia
EMN12/HOVON129 Study Phase 2: To evaluate progression-free survival in adult pPCL patients by incorporation of carfilzomib and...
Dec 9, 2015
111


NCT02199665: Phase 1: Selinexor, Carfilzomib, & Dexamethasone in Treating Patients With RRMM (SINE)
Multiple Myeloma Research Consortium NCT02199665: Phase 1: Selinexor, Carfilzomib, and Dexamethasone in Treating Patients With Relapsed...
Dec 13, 2014
112


Prospective Research Assessment in Multiple Myeloma: An Observational Evaluation (PREAMBLE)
Prospective Research Assessment in Multiple Myeloma: An Observational Evaluation (PREAMBLE) The purpose of this study is to assess the...
Dec 12, 2013
88


NCT01665794: Phase 1/2: Carfilzomib, Pomalidomide, and Dexamethasone in Treating Patients With RRMM
NCT01665794: Phase 1/2: Carfilzomib, Pomalidomide, and Dexamethasone in Treating Patients With Relapsed or Refractory Multiple Myeloma...
Dec 21, 2012
164

