

NCT03290950: Phase 2 - A Study of Daratumumab in Patients With NDMM wKRd-D - Manhattan Trial
MANHATTAN TRIAL NCT03290950: Phase 2 - A Study of Daratumumab in Patients With Newly Diagnosed Multiple Myeloma Dara-KRd A Study of...
Dec 31, 2017
795


NCT03277105: Phase 3 - Subcutaneous vs Intravenous daratumumab in relapsed MM - COLUMBA study
COLUMBA study A Study of Subcutaneous Versus (vs.) Intravenous Administration of Daratumumab in Participants With Relapsed or Refractory...
Dec 29, 2017
320


NCT03274219 : Phase 1 - bb21217 an Anti-BCMA CAR T Cell Drug Product, in RRMM - CRB-402
CRB-402 Study of bb21217 in Multiple Myeloma Relapsed Refractory Multiple Myeloma RRMM Study CRB-402 is a 2-part, non-randomized, open...
Dec 29, 2017
423


NCT03180736 : Phase 3 - EMN 14 - Pomalidomide and Dexa +/- Dara in relapsed MM - APOLLO study
The APOLLO study EMN14/54767414MMY3013 Comparison of Pomalidomide and Dexamethasone With or Without Daratumumab in Subjects With Relapsed...
Dec 28, 2017
336


NCT03158688: Phase 3 - Carfilzomib, Daratumumab and Dexa for relapsed Multiple Myeloma. (CANDOR)
Phase 3 CANDOR study Dara-Kd Kd Study of Carfilzomib, Daratumumab and Dexamethasone for Patients With Relapsed and/or Refractory Multiple...
Dec 28, 2017
343


NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy RRMM Myeloma
NCT03031730: Phase 1: Testing the Addition of KRT-232 (AMG 232) to Usual Chemotherapy for Relapsed Multiple Myeloma Testing the Addition...
Dec 26, 2017
67


NCT03090659: Phase 1/2 - A Chinese study of LCAR-B38M CAR-T cells in RRMM -Myeloma (LEGEND-2)
This is a single arm, open-label, multi-center, phase 1/2 study, to determine the safety and efficacy of LCAR-B38M CAR-T cells in...
Dec 22, 2017
296


Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone
Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone in multiple myeloma: A systematic review...
Dec 22, 2017
24


FDA Approved for Maintenance: Lenalidomide (Revlimid)
On February 22, 2017, the U.S. Food and Drug Administration approved lenalidomide (Revlimid, Celgene Corp.) as maintenance therapy for...
Dec 17, 2017
48


NCT03151811: Phase 3- Melphalan Flufenamide (Melflufen)-Dex or Pom-dex for RRMM ref. to Len. (OCEAN)
OCEAN NCT03151811: Phase 3: A Study of Melphalan Flufenamide (Melflufen)-Dex or Pomalidomide-dex for RRMM Patients Refractory to...
Dec 17, 2017
211

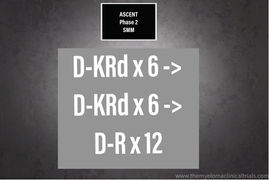
NCT03289299 : Phase 2 - Agg. Smoldering Curative Approach Eval. Novel Therapies & Transplant ASCENT
ASCENT TRIAL Dara-KRd Aggressive Smoldering Curative Approach Evaluating Novel Therapies and Transplant (ASCENT) This study evaluates the...
Dec 16, 2017
442


NCT03319667: Phase 3 - Isatuximab, Bortezomib, Lenalidomide and Dexamethasone NDMM (IMROZ)
IMROZ Study NCT03319667 Clinical Benefit of SAR650984, Bortezomib, Lenalidomide and Dexamethasone Combination in NDMM Patients Not...
Dec 16, 2017
664


NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed Refractory Myeloma
Multiple Myeloma Research Consortium NCT03091257: Phase 1 - Dabrafenib and/or Trametinib in Patients With Relapsed and/or Refractory...
Dec 15, 2017
131


NCT03106324: Safety Study of Lenalidomide in Previously Untreated Adult Multiple Myeloma -TNE
NCT03106324: Safety Study of Lenalidomide in Previously Untreated Adult Multiple Myeloma Patients Who Are Not Eligible for Transplant A...
Dec 15, 2017
67


NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab MGUS / Smoldering Myeloma SMM
NCT03236428: Phase 2: Phase II Study of the CD38 Antibody Daratumumab in Patients With High-Risk MGUS and Low-Risk Smoldering Multiple...
Dec 15, 2017
179


NCT03374085: Phase 1/2: CC-92480 Monotherapy and with Dex in Relapsed / Refractory Multiple Myeloma
NCT03374085: Phase 1/2: CC-92480 Monotherapy and with Dex in Relapsed / Refractory Multiple Myeloma A Safety, PK and Efficacy Study of...
Dec 15, 2017
282


FDA Approved for RRMM: Daratumumab + Pomalidomide & Dexamethasone
HORSHAM, PA, June 16, 2017 – Janssen Biotech, Inc. announced today that the U.S. Food and Drug Administration (FDA) has approved the...
Dec 14, 2017
57


Myeloma Canada: Multiple Myeloma Patient Handbook
Myeloma Canada: Multiple Myeloma Patient Handbook https://myeloma.ca/pixms/uploads/serve/ckeditor/myeloma_canada_patient_handbook_10_2017...
Dec 14, 2017
173


Phase II HOVON 143 Study: Ixazomib, Dara and Low Dose Dex in Intermediate-Fit Patients NDMM
Phase II HOVON 143 Study Trial Update 63rd ASH Annual Meeting 80 Ixazomib, Daratumumab and Low Dose Dexamethasone in Intermediate-Fit...
Dec 14, 2017
282

